About Us
Surgitools Medical Instruments Co., Ltd.
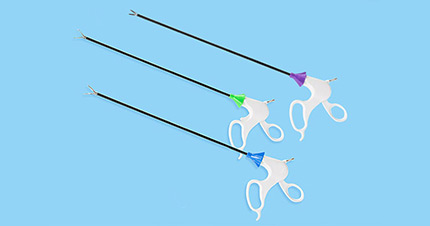
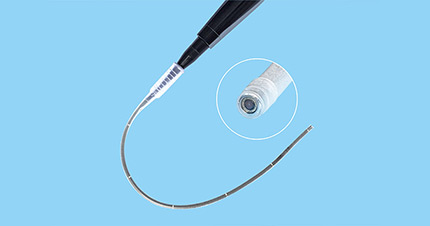
Surgitools has been developing, producing and marketing innovative products for healthcare professionals in minimally invasive surgery. We offer diverse portfolio of high-quality devices, supplies and services for the global electrosurgery and endosurgery markets. Surgitools has evolved to be a leading MIS solution provider of innovative surgical products worldwide.
Our mission is to help medical professionals around the world to improve the quality of healthcare by developing, manufacturing, and marketing innovative, quality medical products. People at Surgitools continuously focus on the safety of patients and the effectiveness of products and services, and emphasize on customer satisfaction.
We strive to build a sustainably growing enterprise by pursuing excellence and exceeding our customers’ expectation in all aspects.

 Self-owned FactoryVisit our 5,000 square feet factory and warehouse.
Self-owned FactoryVisit our 5,000 square feet factory and warehouse.
 Quality ManagementCE, EN-ISO 13485:2016, U.S. Food and Drug Administration (FDA).
Quality ManagementCE, EN-ISO 13485:2016, U.S. Food and Drug Administration (FDA).
 Customized ServiceProvide professional technical and production solutions for minimally invasive surgery.
Customized ServiceProvide professional technical and production solutions for minimally invasive surgery.
 Quality Control ProcessWhich can independently complete microbial limit test, EO/ECH residue test, product/BI sterility test and other related tests.
Quality Control ProcessWhich can independently complete microbial limit test, EO/ECH residue test, product/BI sterility test and other related tests.
Surgitools Medical Instruments Co., Ltd.
Surgitools has been developing, producing and marketing innovative products for healthcare professionals in minimally invasive surgery. We offer diverse portfolio of high-quality devices, supplies and services for the global electrosurgery and endosurgery markets. Surgitools has evolved to be a leading MIS solution provider of innovative surgical products worldwide.
Our mission is to help medical professionals around the world to improve the quality of healthcare by developing, manufacturing, and marketing innovative, quality medical products. People at Surgitools continuously focus on the safety of patients and the effectiveness of products and services, and emphasize on customer satisfaction.
We strive to build a sustainably growing enterprise by pursuing excellence and exceeding our customers’ expectation in all aspects.

 Self-owned FactoryVisit our 5,000 square feet factory and warehouse.
Self-owned FactoryVisit our 5,000 square feet factory and warehouse.
 Quality ManagementCE, EN-ISO 13485:2016, U.S. Food and Drug Administration (FDA).
Quality ManagementCE, EN-ISO 13485:2016, U.S. Food and Drug Administration (FDA).
 Customized ServiceProvide professional technical and production solutions for minimally invasive surgery.
Customized ServiceProvide professional technical and production solutions for minimally invasive surgery.
 Quality Control ProcessWhich can independently complete microbial limit test, EO/ECH residue test, product/BI sterility test and other related tests.
Quality Control ProcessWhich can independently complete microbial limit test, EO/ECH residue test, product/BI sterility test and other related tests.



 molly@surgitoolssh.com
molly@surgitoolssh.com